Tongee Medical’s Another Scientific Research Achievement was Granted European Invention Patent to Drive High-quality Development of Medical Device Industry with Innovation
Introduction:
Recently, “Gastric diverter and digestive tract support and release method”, independently developed by Tongee Medical Technology Co., Ltd. (“Tongee Medical”), was officially patented by the European Patent Office. This scientific research innovation was led and completed by Tongee, which owns its complete independent intellectual property rights. Meanwhile, the applications for the patent portfolio of Tongee Medical’s other inventions have been started simultaneously in the United States, European countries, Japan, Russia and other countries.
As indicated in “Global Adult Body Weight Survey Report” [1] published in Lancet, a well-known British medical journal, in June 2021, the incidence of obesity-associated metabolic disease was on the rise, and there was a huge unmet clinical need for its prevention and treatment [2]. According to statistics from IDF and many domestic literatures, Type 2 diabetes is the most common type of diabetes, accounting for around 90% of all diabetes across the world [3]. Furthermore, overweight and obesity are important environmental factors contributing to the occurrence of Type 2 Diabetes Mellitus (T2DM), as about 90% of T2DM patients suffer from overweight or obesity [4]. According to Frost & Sullivan’s report, the global medical market size for T2DM alone has reached US$70 billion.
The patented gastric diversion support system self-developed by Tongee Medical is one of the innovative technologies in the field of metabolic diseases. As of the end of July 2022, Tongee has nearly 10 projects under research, more than 50 applications for patents domestically and abroad, and more than 30 patents granted in 18 countries in Europe, America, Canada and Australia. This is the 7th related international patent granted for this project.
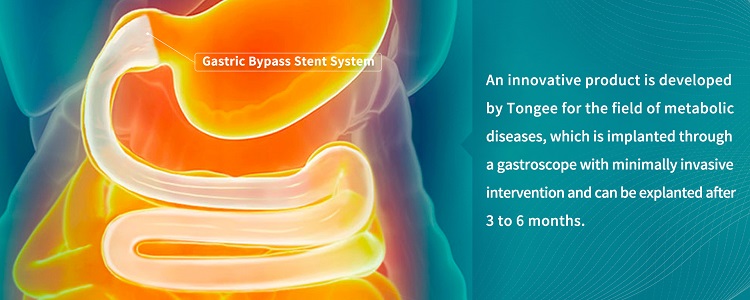
By combining the concept of gastric bypass metabolic surgery with the implantation of an innovative medical device into the digestive tract, this breakthrough minimally invasive technology achieves similar effect as the traditional metabolic surgery. It avoids the irreversible damage to the physical structure caused by a surgical operation and postoperative complications, since this medical device is removable after implantation and is easy to operate, providing a new path for the treatment of patients suffering from metabolic diseases such as obesity, Type 2 Diabetes and non-alcoholic fatty liver disease.
Currently, Tongee Medical’s gastric diversion support system is in the Special Examination and Approval Procedures for Innovative Medical Devices in China and has been recognized as a Class III innovative medical device by National Medical Products Administration. At present, this medical device has successfully gone through the critical phases of device therapy and removal in the clinic trials, and enters into the later follow-up phase.
References:
[1] Xiong-Fei Pan, et al. Epidemiology and determinants of obesity in China. The Lancet Diabetes and Endocrinology VOLUME 9, ISSUE 6, P373-392, JUNE 01, 2021
[2] IDF Diabetes Atlas (10th Edition) [J].Chinese Journal of Diabetes Mellitus, 2021:12-6.
[3] New Progress in Clinical Treatment of Type 2 Diabetes [J]. Chinese Journal of Medical Device, 2019, 32(24): 203-204.
[4] Chinese Journal of Endocrinology and Metabolism, 2004, 20(5): 5.
